Abstract
Background: Patients with CLL are among the highest risk population of SARS-CoV-2 infection attributed to disease and therapy. Patients receiving treatment, particularly after recent anti-CD20 monoclonal antibodies therapy, have low seroconversion rates after mRNA COVID-19 vaccine. An emergent recommendation of preexposure prophylaxis with monoclonal-antibody combination AZD7442 in immunocompromised patients is based on a trial conducted before the emergence of the BA.1 (Omicron) and later variants and did not include patients with CLL.
Methods: Patients with CLL tested for serologic response and neutralization effect of COVID-19 variants Wild Type (WT), Delta, Omicron (BA.1) BA.2, BA.4 and BA.5 , before and after a single dose of monoclonal-antibody combination AZD7442 (150 mg of tixagevimab and 150 mg of cilgavimab) administration.
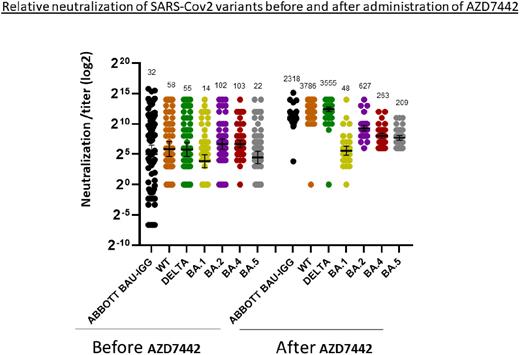
Results: Fifty patients (31 males/ 19 females) with CLL, median age 62 (range 38 to 81 years) were included in the study. Their baseline characteristics include CIRS score 6 (IQR, 4-8), IGHV unmutated 62% (15/24), Dohner scale (del13q 40%, Normal 16% del11q 9%, T12 18% , del17p/TP53mut 17%) and hypogammaglobulinemia 88% (42/48) with low IgG 65%, low IgA 54% and low IgM 79%. Thirty percent (15/50) were therapy naïve and 54% (27/50) received therapy with ongoing BTKi 30% (15/50) and ongoing BCL2i 20% (10/50), other therapies 4% (2). Fifty percent of patients (25/50) received monoclonal anti-CD20 antibodies (ab), of which 28% ≥12 month from AZD7442 and 22% <12 months from AZD7442. In this group, 3 patients received ongoing anti-CD20 ab. Four patients had previous COVID19 infection within 3 months from AZD7442. Baseline serology was tested 12 days (IQR, 4-22 days) before AZD7442 administration, and serologic response was tested 19 days (IQR,16-25 days) after AZD7442 administration. Anti-spike abs increased from 14 (IQR, 0.4-284) to 2308 (IQR, 1644-3219) BAU/ml. While only 56% (28/50) of patients had positive serology (>21.4 BAU/ml) before AZD7442, 98% (49/50) had positive serology after AZD7442. Viral neutralization of WT and Delta variants before and after AZD7442 increased 65 folds, and for BA.1 (Omicron), BA.2, BA.4 and BA.5 increased 3.4, 6.1, 2.5 and 9.5 folds respectively (figure 1). Over follow-up period of 3 months, 13 patients with CLL (26%) acquired COVID-19 infection, none of them required hospitalization and all recovered uneventfully. There was no significant statistical difference in neutralization ab levels between infected and uninfected patients for the prevalent variant at that time (BA.5). Time from previous mRNA vaccination and ongoing assessment of infected vs uninfected patients is being further evaluated.
Conclusion: After receiving preexposure prophylaxis with AZD7442, patients with CLL have increased IgG antibodies titer to SARS-CoV-2 more than seventy folds higher. Neutralization effect to WT and Delta variants is much higher than Omicron, BA.2, BA.4 and BA.5 variants. The effectivity of a double dose of AZD7442 or more specific monoclonal antibodies to the prevalent variants need to be further evaluated.
Disclosures
Benjamini:AbbVie,Janssen,Astrazeneca: Consultancy. Tadmor:Janssen: Research Funding; AbbVie, Roche, Novartis, Sanofi, Takeda, Janssen, Pfizer: Consultancy, Honoraria, Speakers Bureau. Avigdor:Takeda, Gilead, Novartis, Roche, BMS: Consultancy; AbbVie: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal